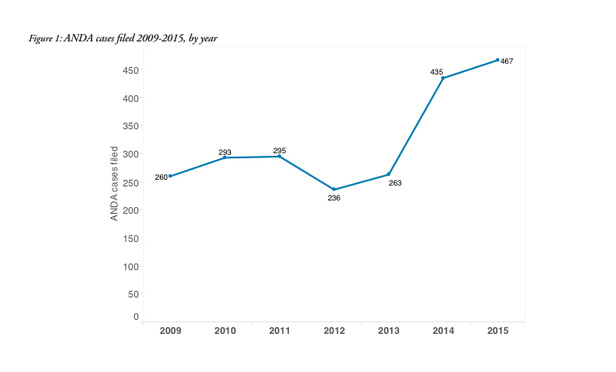
The number of patent litigation filings related to new drug applications before the U.S. Food and Drug Administration, commonly known as ANDA (Abbreviated New Drug Applications) filings, increased 68 percent in 2014-15 compared with the average number of cases filed in each of the previous five years, a new report by the legal analytics company Lex Machina says.
Between 2009 and 2013, an average of 269 cases were filed each year, but that average rose to 451 in 2014-15, according to the report, which is to be released Tuesday and covers the 2,249 ANDA cases filed in U.S. district courts between Jan. 1, 2009, and Dec. 31, 2015.
This content has been archived. It is available through our partners, LexisNexis® and Bloomberg Law.
To view this content, please continue to their sites.
Not a Lexis Subscriber?
Subscribe Now
Not a Bloomberg Law Subscriber?
Subscribe Now
LexisNexis® and Bloomberg Law are third party online distributors of the broad collection of current and archived versions of ALM's legal news publications. LexisNexis® and Bloomberg Law customers are able to access and use ALM's content, including content from the National Law Journal, The American Lawyer, Legaltech News, The New York Law Journal, and Corporate Counsel, as well as other sources of legal information.
For questions call 1-877-256-2472 or contact us at [email protected]