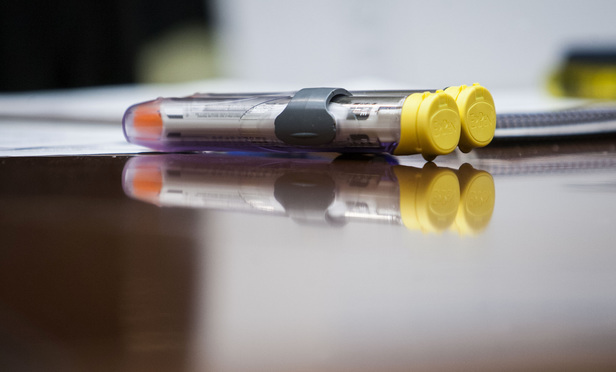
Just before the close of business last Friday, Mylan N.V. announced it would pay $465 million to the U.S. Justice Department and other agencies to resolve regulatory questions about the company’s alleged misclassification of the EpiPen device for purposes of Medicaid rebates.
Three federal agencies have remained mum about the settlement—announced on the eve of a federal holiday and during the media blitz over Donald Trump’s lewd remarks about women—because it has not been finalized. Mylan, and not the government, broke the news about its deal.
This content has been archived. It is available through our partners, LexisNexis® and Bloomberg Law.
To view this content, please continue to their sites.
Not a Lexis Subscriber?
Subscribe Now
Not a Bloomberg Law Subscriber?
Subscribe Now
LexisNexis® and Bloomberg Law are third party online distributors of the broad collection of current and archived versions of ALM's legal news publications. LexisNexis® and Bloomberg Law customers are able to access and use ALM's content, including content from the National Law Journal, The American Lawyer, Legaltech News, The New York Law Journal, and Corporate Counsel, as well as other sources of legal information.
For questions call 1-877-256-2472 or contact us at [email protected]