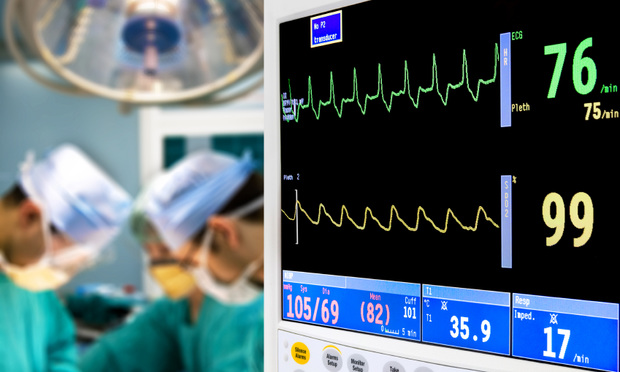
The U.S. Food and Drug Administration is not planning to enforce regulations about devices that store and display medical data because they pose a low risk to patients, according to a draft guidance released last week.
Examples of medical device data systems include the software that displays a previously stored electrocardiogram and software that collects the output from a ventilator about a patient’s carbon dioxide levels and transmits the information to a centralized data repository, according to the agency.
This content has been archived. It is available through our partners, LexisNexis® and Bloomberg Law.
To view this content, please continue to their sites.
Not a Lexis Subscriber?
Subscribe Now
Not a Bloomberg Law Subscriber?
Subscribe Now
LexisNexis® and Bloomberg Law are third party online distributors of the broad collection of current and archived versions of ALM's legal news publications. LexisNexis® and Bloomberg Law customers are able to access and use ALM's content, including content from the National Law Journal, The American Lawyer, Legaltech News, The New York Law Journal, and Corporate Counsel, as well as other sources of legal information.
For questions call 1-877-256-2472 or contact us at [email protected]