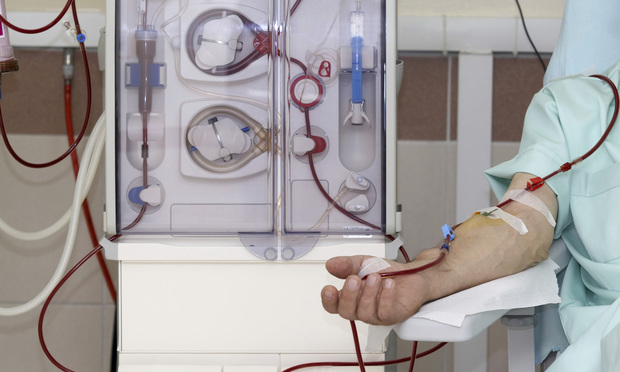
A manufacturer of dialysis machines and related products should have to disclose what it knew about “adverse events,” plaintiffs argue in federal multidistrict litigation pending in Massachusetts.
Fresenius Medical Care Holdings Inc. argues the information is irrelevant, but “courts in products-liability, failure-to-warn and defect cases regularly require the defendants to produce adverse-event information, recognizing that such information is relevant, indeed key, to the issue of whether the manufacturer had notice and knowledge of the defect in its product and adequately warned,” the plaintiffs said in a court filing.
This content has been archived. It is available through our partners, LexisNexis® and Bloomberg Law.
To view this content, please continue to their sites.
Not a Lexis Subscriber?
Subscribe Now
Not a Bloomberg Law Subscriber?
Subscribe Now
LexisNexis® and Bloomberg Law are third party online distributors of the broad collection of current and archived versions of ALM's legal news publications. LexisNexis® and Bloomberg Law customers are able to access and use ALM's content, including content from the National Law Journal, The American Lawyer, Legaltech News, The New York Law Journal, and Corporate Counsel, as well as other sources of legal information.
For questions call 1-877-256-2472 or contact us at [email protected]