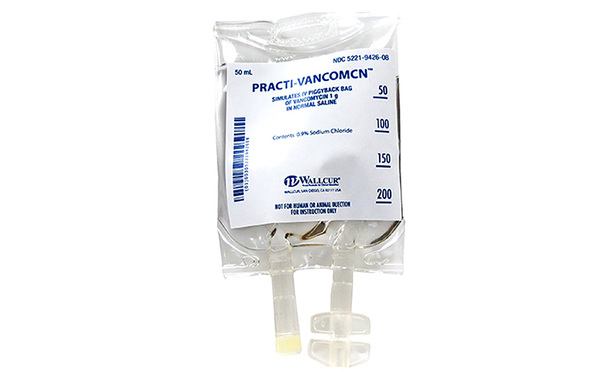
The U.S. Food and Drug Administration and the Centers for Disease Control and Prevention are investigating the infusions of non-sterile, simulated intravenous saline to patients in seven states, including some who were hospitalized and at least one person who died.
The simulated saline products, made by San Diego-based Wallcur LLC for training purposes only, were administered to more than 40 patients in Florida, Georgia, Idaho, Louisiana, North Carolina, New York, and Colorado, the FDA said in a Jan. 14 safety alert. The FDA warned these products are not sterile and should not be injected in humans or animals.
This content has been archived. It is available through our partners, LexisNexis® and Bloomberg Law.
To view this content, please continue to their sites.
Not a Lexis Subscriber?
Subscribe Now
Not a Bloomberg Law Subscriber?
Subscribe Now
LexisNexis® and Bloomberg Law are third party online distributors of the broad collection of current and archived versions of ALM's legal news publications. LexisNexis® and Bloomberg Law customers are able to access and use ALM's content, including content from the National Law Journal, The American Lawyer, Legaltech News, The New York Law Journal, and Corporate Counsel, as well as other sources of legal information.
For questions call 1-877-256-2472 or contact us at [email protected]