Report: New Medical, Pharma Filings in Federal Courts Declined
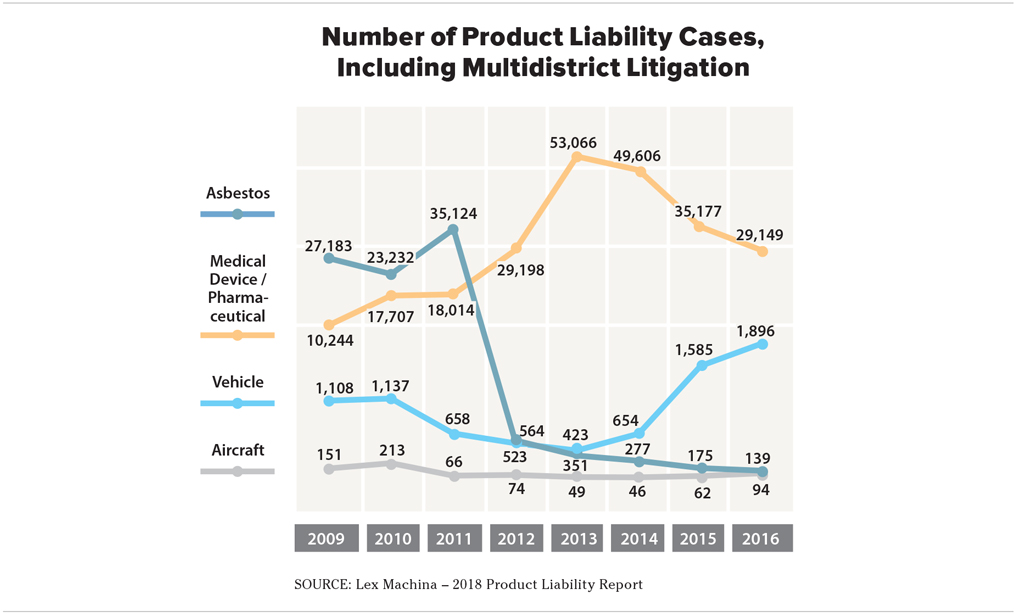
In the same timeframe, new filings for vehicle liability cases grew from 423 cases to 1,896 in 2016. Aircraft liability case filings ratcheted up from 49 cases to 94.
April 02, 2018 at 12:43 PM
4 minute read

Medical device and pharmaceutical liability lawsuit filings in federal courts, while still the leading category of products liability complaints, have declined slightly in recent years while vehicle and aircraft cases are on the rise, according to a new report released Monday.
In 2016, there were 29,149 medical device and pharmaceutical liability cases filed in federal courts, including multidistrict litigation, which makes up the overwhelming majority of products liability cases filed in federal courts.
That's a 45 percent drop from 2013, when new cases spiked to 53,066 from 29,198 the previous year, according to a report on trends in products liability litigation by Lex Machina, a legal analytics firm.
In the same timeframe, new filings for vehicle liability cases grew from 423 cases to 1,896 in 2016. Aircraft liability case filings ratcheted up from 49 cases to 94.
Dennis Raglin of San Francisco-based Rimon PC, who represents defendants in products liability cases, said the recent decline in medical product and pharmaceutical cases could be due to the fact that case filings of that type tend to come in “waves.”
He said that a recent spike in lawsuits against pharmaceutical companies over their supposed role in the spread of the opioid epidemic, such as the roughly 400 lawsuits filed in federal court in Ohio against drug manufacturers and distributors, may drive the numbers back up.
“It could break new ground on what happens in product liability because it's the first case that really tests the waters,” Raglin said of the Ohio opioid litigation.
The report did not include cases that dealt solely with claims of false advertising, negligent operation or installation or negligence against medical staff and health care providers.
According to the report, when excluding MDL cases, the powerhouses of all products liability litigation filings from 2009 to 2017 are the U.S. district courts in the Central District of California, the Eastern District of Pennsylvania and the District of New Jersey, which collectively accounted for 15 percent of all non-MDL products liability cases filed.
As for medical product and pharmaceutical cases, also excluding MDL cases, the top three courts were California's Central District, the District of New Jersey and the Eastern District of Louisiana; the three courts accounted for 22 percent of new filings of cases of that type.
With regard to how the federal district courts resolve the cases, the vast majority of medical device and pharmaceutical products liability cases are settled or resolved procedurally, the report states.
But when those issues are resolved on the merits of the case, the defense bar wins 90 percent of the time, according to the report.
The top firms representing plaintiffs in medical device and pharmaceutical cases that were terminated in 2016 or 2017—which tend to be small, products liability boutiques—are the St. Louis-based Carey Danis & Lowe; the California-based Knox Ricksen; Schuckit & Associates, which is based in Indiana; Lopez McHugh, based in New Jersey; and Holland & Hart of Colorado.
On the defense side, the top products liability firms tend to be larger and more nationally based. They were Shook, Hardy & Bacon; Reed Smith; Greenberg Traurig; Ice Miller; and Faegre Baker Daniels.
Of all product types, asbestos case filings saw the most significant decline. There were 35,124 asbestos cases filed in federal courts in 2011, which fell to 564 in 2012 and steadily declined to 139 cases in 2016.
The decline in asbestos case filings mirrors the trend in state courts across the country.
This content has been archived. It is available through our partners, LexisNexis® and Bloomberg Law.
To view this content, please continue to their sites.
Not a Lexis Subscriber?
Subscribe Now
Not a Bloomberg Law Subscriber?
Subscribe Now
NOT FOR REPRINT
© 2025 ALM Global, LLC, All Rights Reserved. Request academic re-use from www.copyright.com. All other uses, submit a request to [email protected]. For more information visit Asset & Logo Licensing.
You Might Like
View All

Family Law Practitioners Weigh In on Court System's New Joint Divorce Program

Former NY City Hall Official Tied to Adams Corruption Probe to Plead Guilty

New Charges Expected in Sex Trafficking Case Against Broker Brothers
Trending Stories
- 1ACC CLO Survey Waves Warning Flags for Boards
- 2States Accuse Trump of Thwarting Court's Funding Restoration Order
- 3Microsoft Becomes Latest Tech Company to Face Claims of Stealing Marketing Commissions From Influencers
- 4Coral Gables Attorney Busted for Stalking Lawyer
- 5Trump's DOJ Delays Releasing Jan. 6 FBI Agents List Under Consent Order
Who Got The Work
J. Brugh Lower of Gibbons has entered an appearance for industrial equipment supplier Devco Corporation in a pending trademark infringement lawsuit. The suit, accusing the defendant of selling knock-off Graco products, was filed Dec. 18 in New Jersey District Court by Rivkin Radler on behalf of Graco Inc. and Graco Minnesota. The case, assigned to U.S. District Judge Zahid N. Quraishi, is 3:24-cv-11294, Graco Inc. et al v. Devco Corporation.
Who Got The Work
Rebecca Maller-Stein and Kent A. Yalowitz of Arnold & Porter Kaye Scholer have entered their appearances for Hanaco Venture Capital and its executives, Lior Prosor and David Frankel, in a pending securities lawsuit. The action, filed on Dec. 24 in New York Southern District Court by Zell, Aron & Co. on behalf of Goldeneye Advisors, accuses the defendants of negligently and fraudulently managing the plaintiff's $1 million investment. The case, assigned to U.S. District Judge Vernon S. Broderick, is 1:24-cv-09918, Goldeneye Advisors, LLC v. Hanaco Venture Capital, Ltd. et al.
Who Got The Work
Attorneys from A&O Shearman has stepped in as defense counsel for Toronto-Dominion Bank and other defendants in a pending securities class action. The suit, filed Dec. 11 in New York Southern District Court by Bleichmar Fonti & Auld, accuses the defendants of concealing the bank's 'pervasive' deficiencies in regards to its compliance with the Bank Secrecy Act and the quality of its anti-money laundering controls. The case, assigned to U.S. District Judge Arun Subramanian, is 1:24-cv-09445, Gonzalez v. The Toronto-Dominion Bank et al.
Who Got The Work
Crown Castle International, a Pennsylvania company providing shared communications infrastructure, has turned to Luke D. Wolf of Gordon Rees Scully Mansukhani to fend off a pending breach-of-contract lawsuit. The court action, filed Nov. 25 in Michigan Eastern District Court by Hooper Hathaway PC on behalf of The Town Residences LLC, accuses Crown Castle of failing to transfer approximately $30,000 in utility payments from T-Mobile in breach of a roof-top lease and assignment agreement. The case, assigned to U.S. District Judge Susan K. Declercq, is 2:24-cv-13131, The Town Residences LLC v. T-Mobile US, Inc. et al.
Who Got The Work
Wilfred P. Coronato and Daniel M. Schwartz of McCarter & English have stepped in as defense counsel to Electrolux Home Products Inc. in a pending product liability lawsuit. The court action, filed Nov. 26 in New York Eastern District Court by Poulos Lopiccolo PC and Nagel Rice LLP on behalf of David Stern, alleges that the defendant's refrigerators’ drawers and shelving repeatedly break and fall apart within months after purchase. The case, assigned to U.S. District Judge Joan M. Azrack, is 2:24-cv-08204, Stern v. Electrolux Home Products, Inc.
Featured Firms
Law Offices of Gary Martin Hays & Associates, P.C.
(470) 294-1674
Law Offices of Mark E. Salomone
(857) 444-6468
Smith & Hassler
(713) 739-1250






