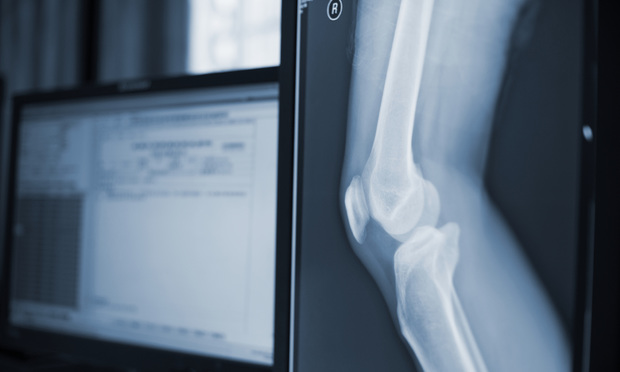
A federal criminal investigation against Stryker subsidiary OtisMed Corp. over sales of an unapproved surgical product concluded Dec. 8 with guilty pleas from the company and its former CEO, along with their agreement to financial penalties that, together with the settlement of a related qui tam action, amount to more than $80 million.
The devices, OtisKnee guides, were used in knee-replacement procedures to assist surgeons in making accurate bone cuts.
This content has been archived. It is available through our partners, LexisNexis® and Bloomberg Law.
To view this content, please continue to their sites.
Not a Lexis Subscriber?
Subscribe Now
Not a Bloomberg Law Subscriber?
Subscribe Now
LexisNexis® and Bloomberg Law are third party online distributors of the broad collection of current and archived versions of ALM's legal news publications. LexisNexis® and Bloomberg Law customers are able to access and use ALM's content, including content from the National Law Journal, The American Lawyer, Legaltech News, The New York Law Journal, and Corporate Counsel, as well as other sources of legal information.
For questions call 1-877-256-2472 or contact us at [email protected]